The SDC Ecosystem
The data driven backbone to support all your clinical trial needs.
SDC DataHub™
SDC DataHub™ is our AI enabled data lake that is the next generation backbone for a Clinical drug development ecosystem.
SDC Insights™
SDC Insights™ provides clients with consolidated access to their data throughout the clinical trial process and beyond.
SDC Capture™
SDC Capture provides an efficient and easy to deploy ePRO/eCOA solution to meet your cost and timelines needs.
SDC DataHub™
SDC DataHub™ is our AI enabled data lake that is the next generation backbone for a Clinical drug development ecosystem.
It allows us to not only centralize data across platforms, but also deploy data out from one system to another to reduce errors and redundancy in data entry. It integrates data from disparate systems allowing us to automate many aspects of our process. It also allows SDC staff to meet shorter timelines with higher quality and enables SDC Insights cross-system reporting functionality. With single sources of truth, we ensure that the information generation comes from the correct source as reports are only as good as the data behind them.
SDC Insights™
SDC Insights™ provides clients with consolidated access to their data throughout the clinical trial process and beyond.
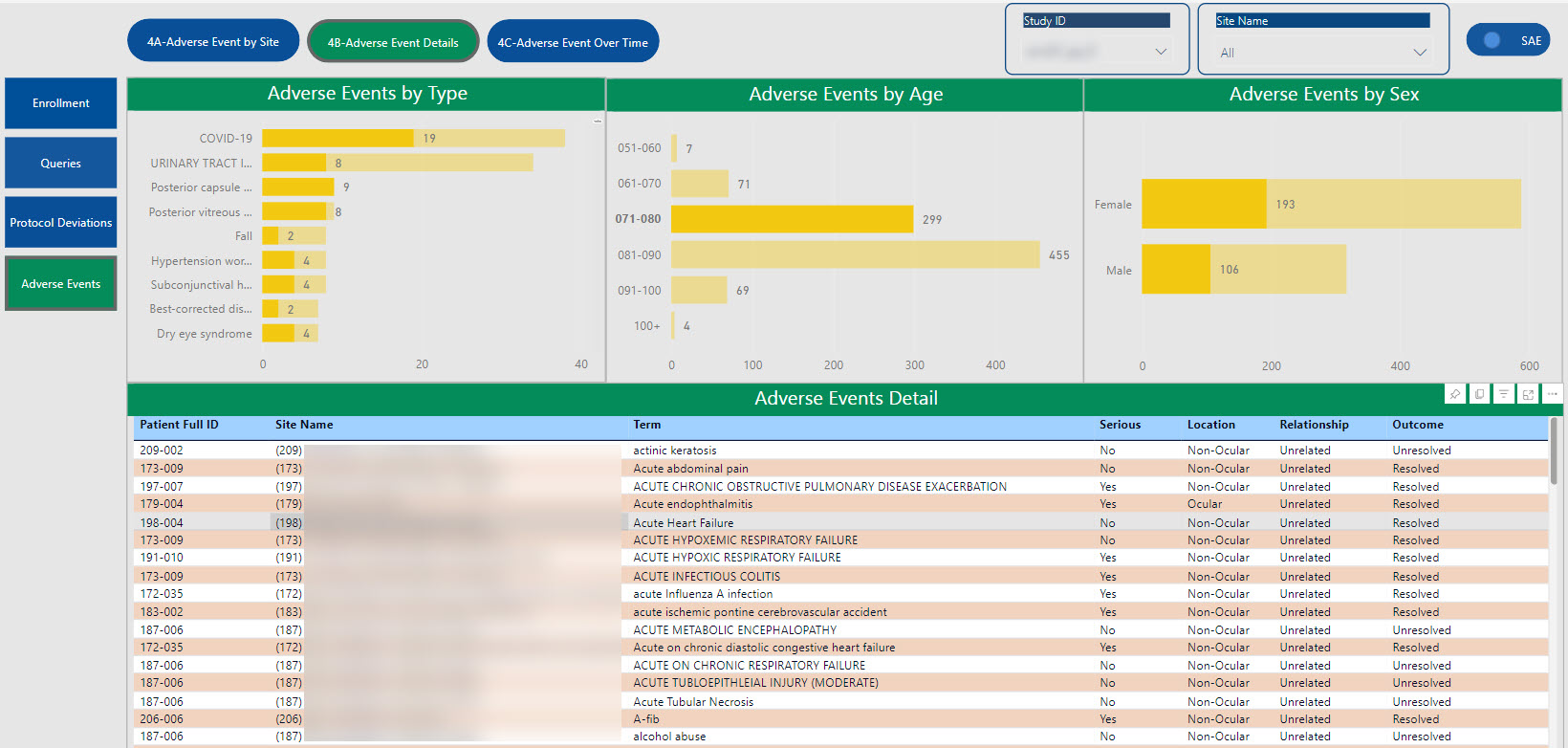
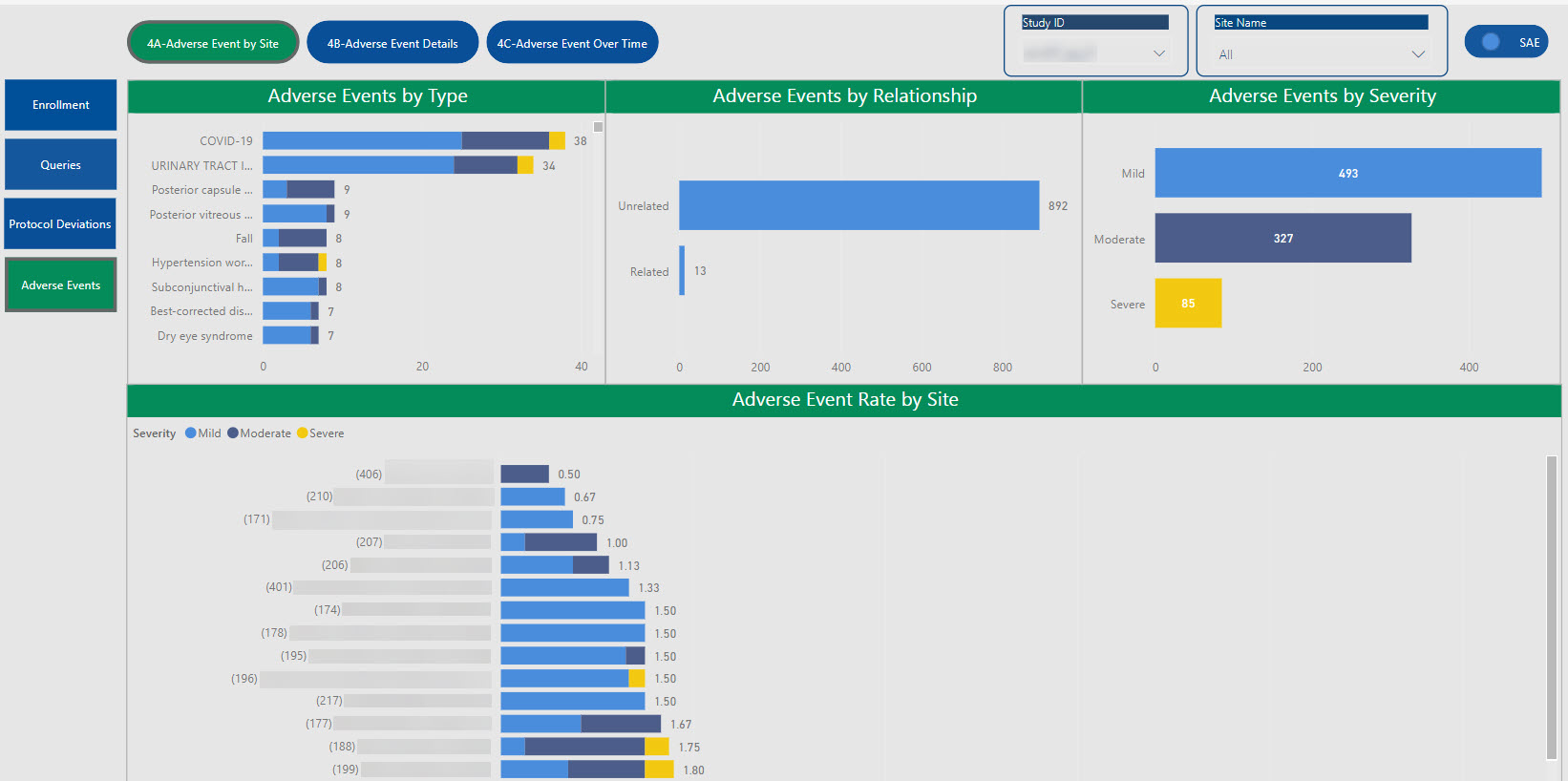
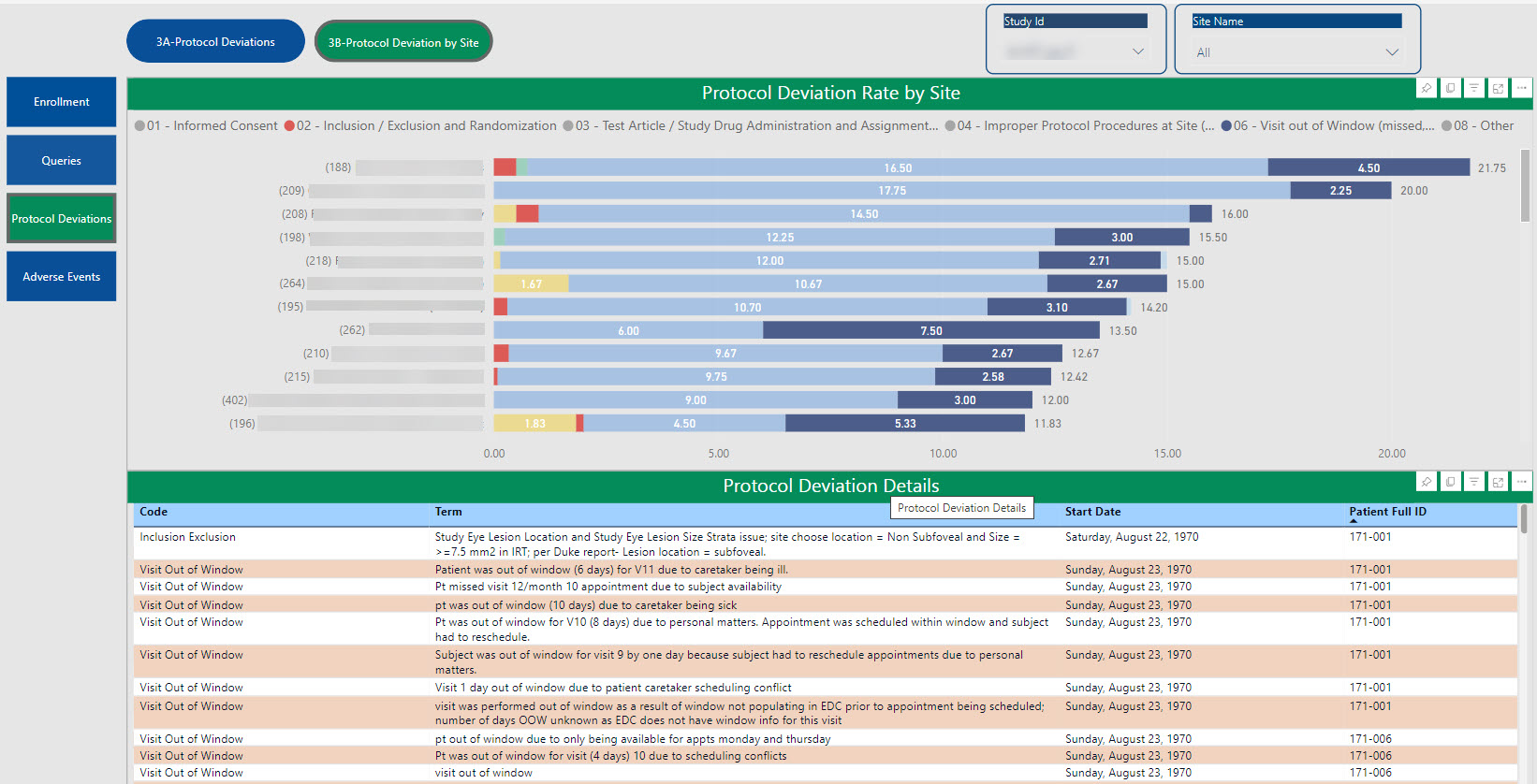
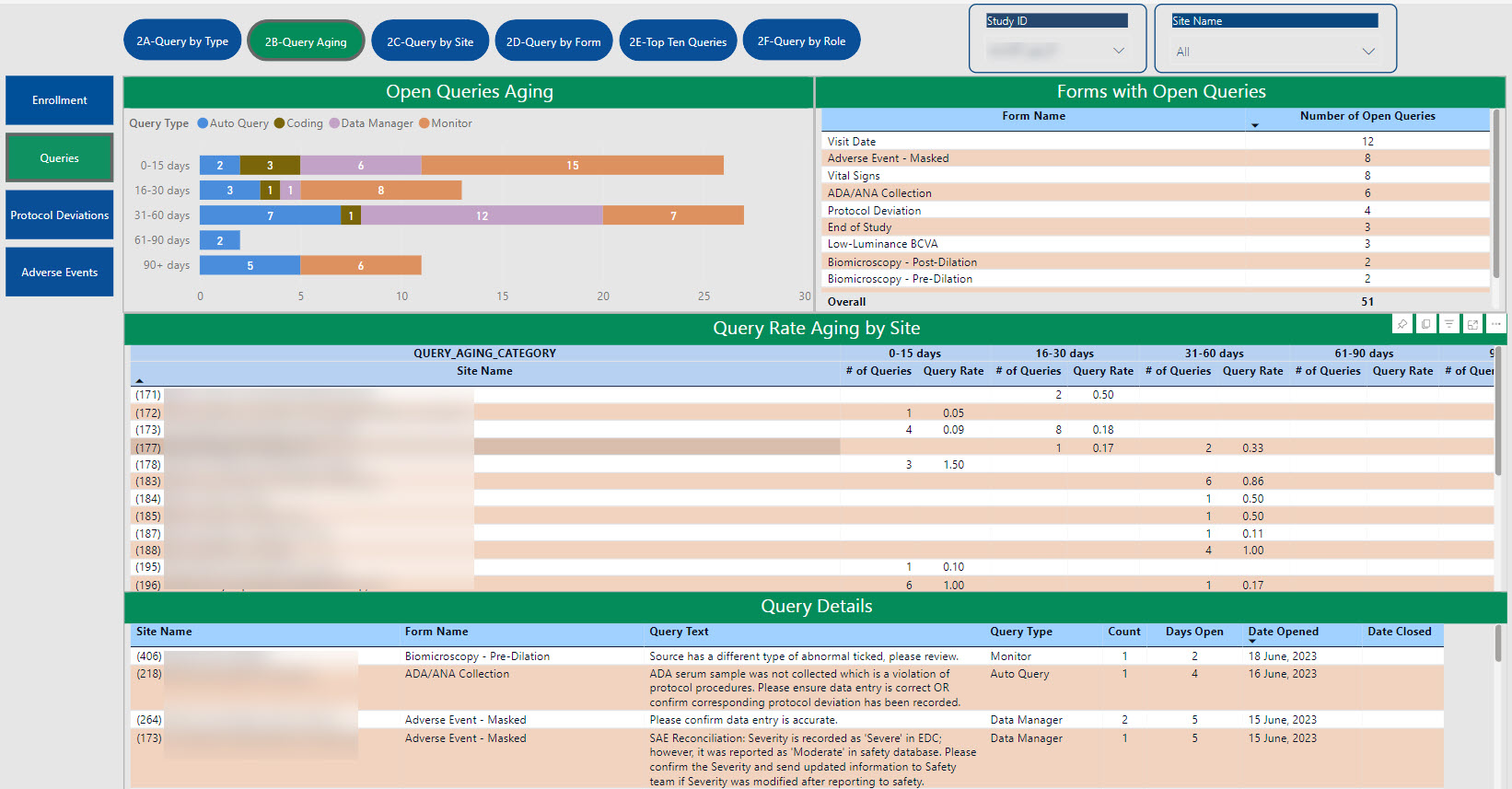
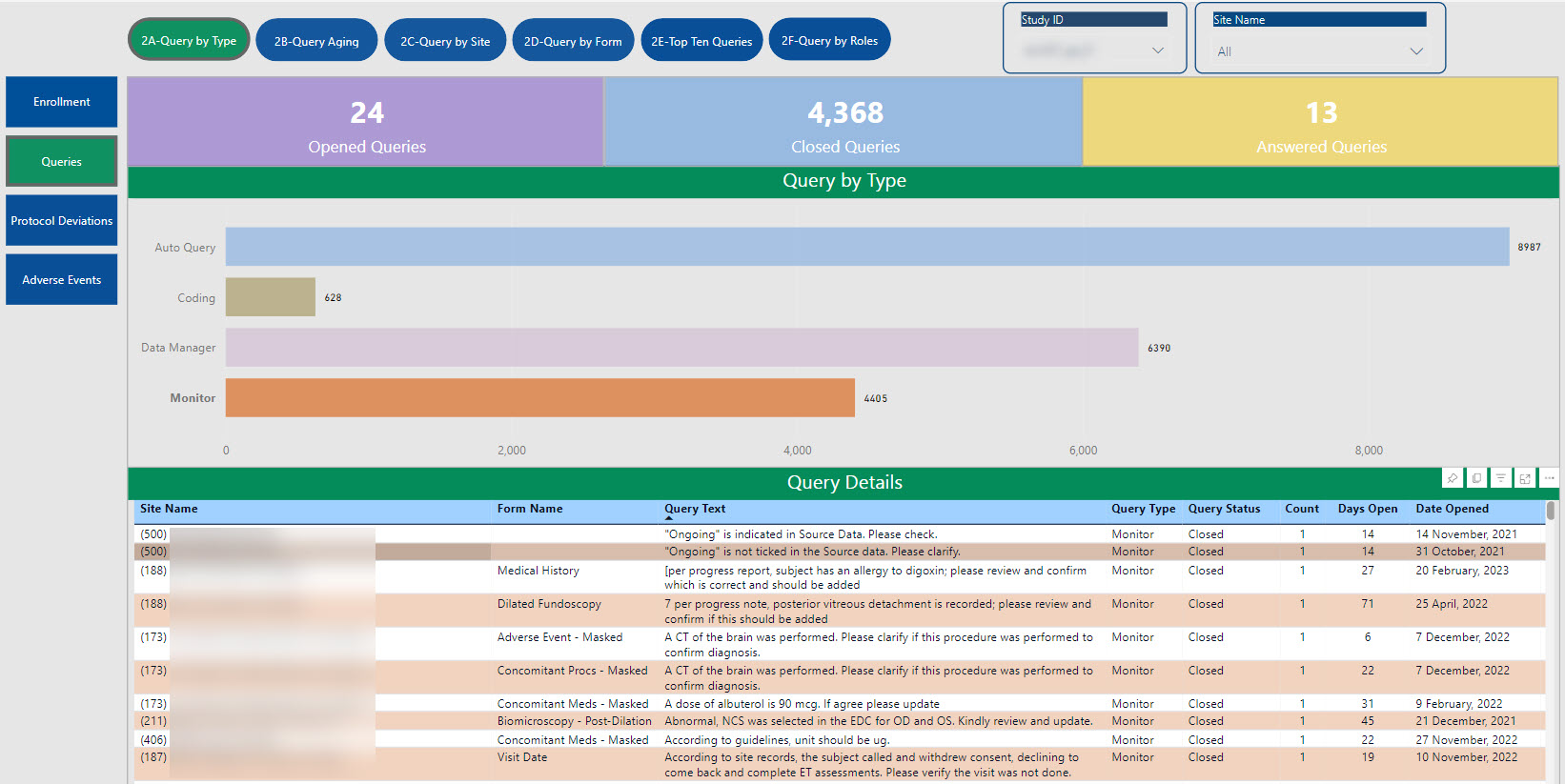
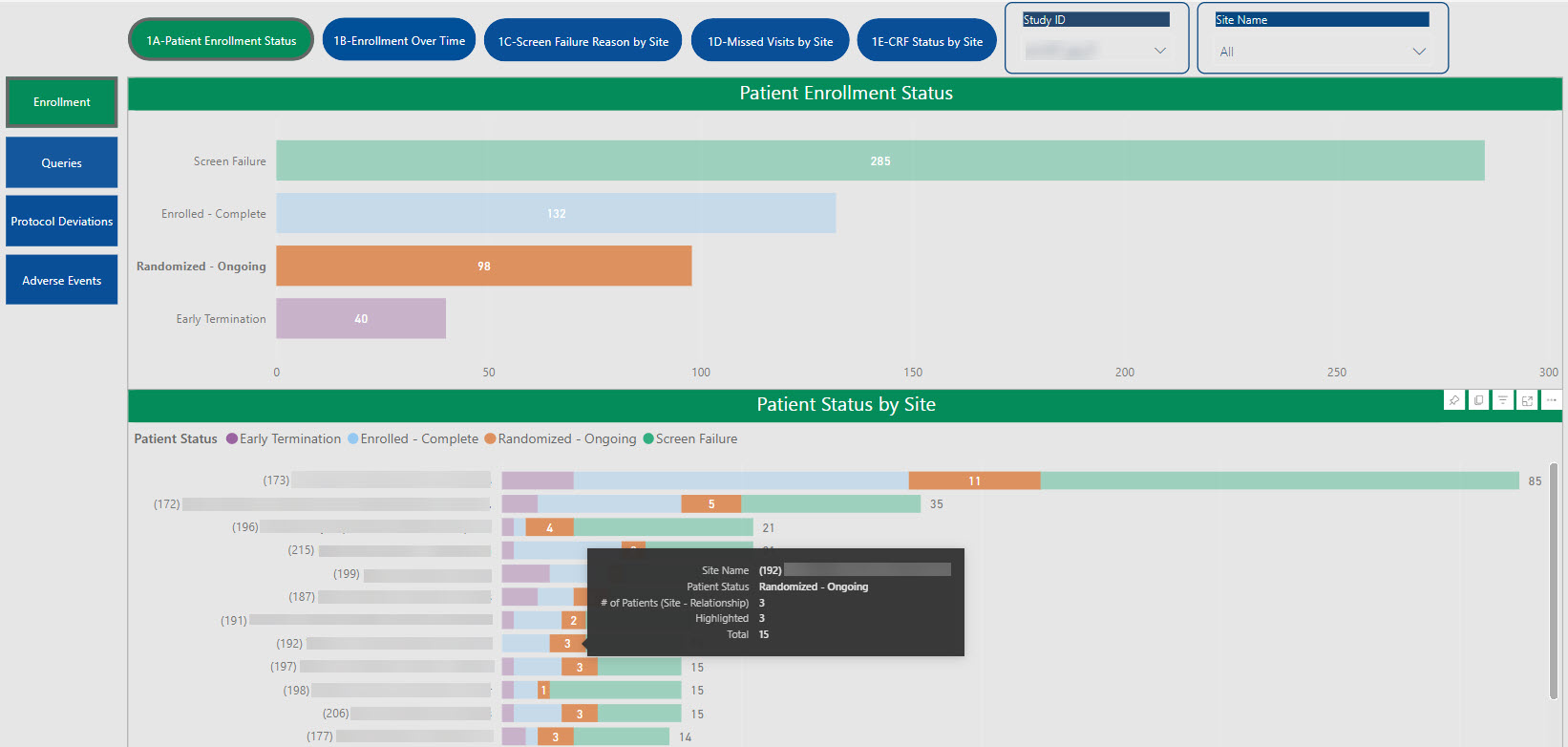
SDC’s robust tool allows for the creation of customized dashboards that pull information from various sources to provide aggregated data, dynamic views, and near real-time reporting.
Data visualizations
Data visualizations provide consolidated reporting, a single source of truth, on a dynamic, living dashboard to monitor study progression and proactively address data trends to valuable study decisions. With cross-system reporting from multiple data sources (CTMS, EDC, IRT, protocol deviations, etc.), the SDC solution helps facilitate communication and timely decision-making as your study progresses and can be accessed by login via the web, PCs, Macs, Android and iOS devices.
The dashboards created for you are able to create a single integrated source for data review, thus providing the ability to monitor and track risk across all platforms in one centralized place.
Data management, Biostatistics, Data Science expertise to help you identify data sources and setup meaningful displays
Customization to meet individual client and study needs
Tools to better support complex studies, clinical development programs, and IP analysis
Single source of truth across studies, independent of who processed the data, which saves time and resources
Trends related to enrollment, data issues, timelines, and expense
SDC Capture™
A flexible ePRO technology
SDC Capture™ is designed to fit around your protocol with workflows and decision trees to support.
SDC Capture™ is a flexible ePRO technology designed for rapid deployment in order to meet both the budgets and timeline demands of modern clinical trials.
SDC Capture™ brings three main capabilities:
Real-time electronic collection of Patient Reported Outcomes
Photo and Video Capture – 21 CFR Part 11 compliant storage
Reminder notifications to ensure protocol compliance
Real time compliance driven payment solutions
SDC Capture™ is designed to fit around your protocol with workflows and decision trees to support. Our ePRO technology has been patient tested to ensure we are able to support a diverse population and improve the patient experience. SDC Capture™ was created with a design in mind to help relieve patient burden and provide a more collaborative experience during your study.
