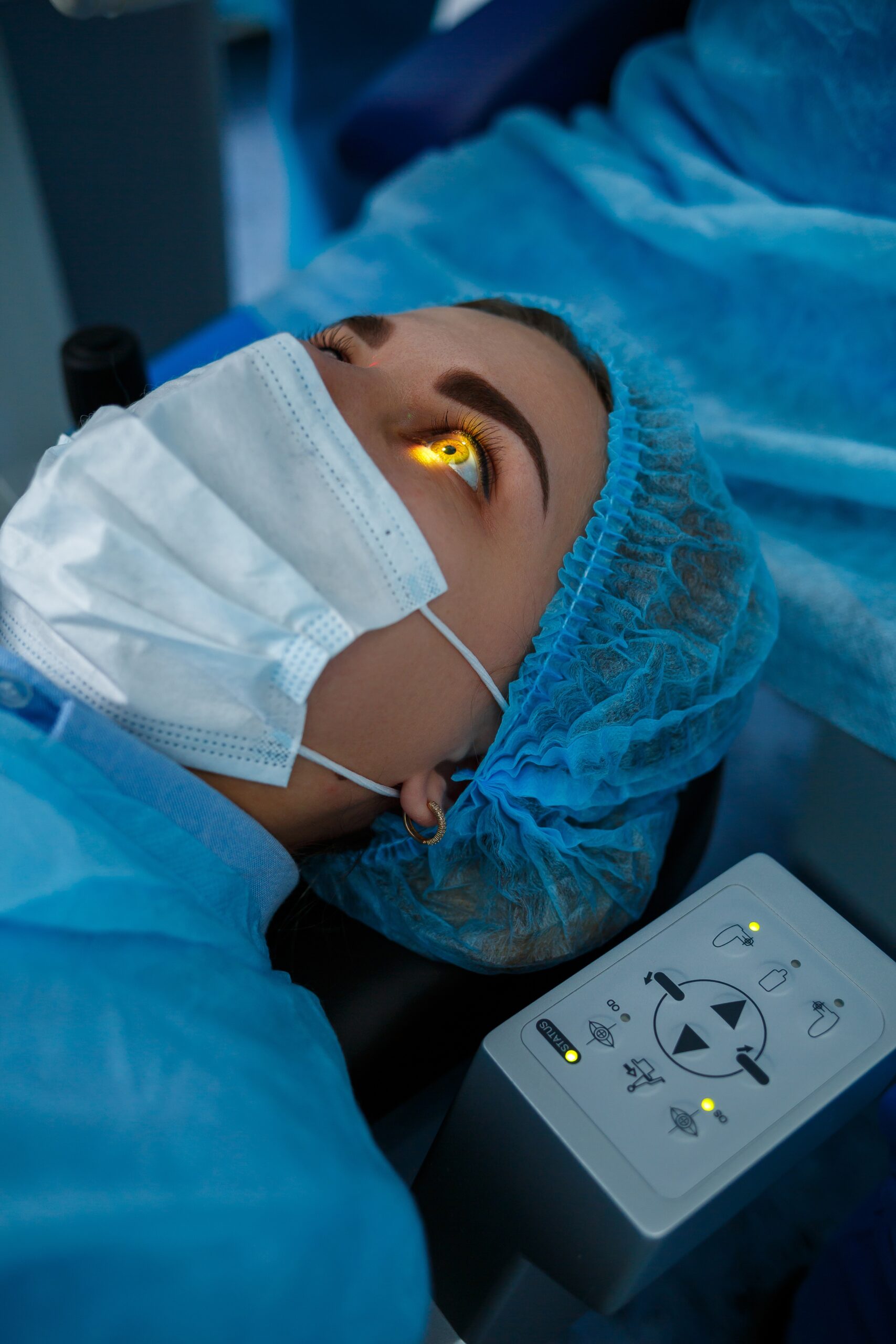
Ophthalmology
30+ Product Approvals and Counting
Supporting over 300+ clinical trials in Ophthalmology, SDC has unparalleled experience in designing, building and analyzing ophthalmic clinical trials.
From Phase 1 to regulatory submission, SDC’s robust ophthalmic experience is The Right Fit For You.
Ophthalmology Experience:
Extensive Ophthalmology experience across the SDC project Teams
All phases of ophthalmic drug development:
Pilot, pivotal, and post-market device and diagnostic trials, including adaptive trial designs and pooled analysis for data submission
30+ Ophthalmology Product Approvals
Frequent interaction with regulatory agencies
Supported development of CDISC standards for ophthalmology
Ophthalmology
30+ Product Approvals and Counting
Oncology
Supporting the Next Generation of Cancer Therapy Through Thoughtful Guidance
Neurology & CNS
Thoughtful Collaboration with Your Team to Guide Your Development Strategy
Ophthalmic Indications
Achromatopsia
AMD: Wet & Dry
Atopic Keratoconjunctivitis
Bacterial Corneal Ulcers
Blepharitis
Conjunctivitis: Allergic, Bacterial, Viral
Contact Lens
Contact Lens Solution
Corneal Deposit
Corneal Incision Sealant
Diabetic Macular Edema (DME)
Diabetic Retinopathy
Dry Eye
Glaucoma: Drug, Device
Impaired Dark Adaptation
Inflammation: Anterior/Posterior Chamber, Post-Operative
Inherited Retinal Disease
Intraocular Lens: Aspheric, Refractive
Leber’s Congenital Amaurosis (LCA)
Lid Scrub
Meibomian Gland Dysfunction
Microkeratome
Neurotrophic Keratopathy
Ocular & Systemic Safety
Ocular Redness
Optical Coherence Tomography Device
Pediatric Cataract
Phacoemulsification
Presbyopia
Retinal Disease
Retinal Vein Occlusion
Retinitis Pigmentosa
Retinoschisis
Specular Microscope
Spheric IOL
Tonometer
Uveitis: Anterior, Posterior
Vitreal Detachment
X-linked Retinoschisis (XLRS)
Ophthalmology
Case Studies
SDC Optimizes Focus on Tech-Enabled Biometric Services
Phoenix, AZ – Statistics & Data Corporation (SDC), a global leader in life sciences biometrics services and technology solutions, announces a strategic decision to enhance focus and efficiency by transitioning its ophthalmic biometric division to its partner Ora,...
SDC Partners with Singular to Advance Secure AI Solutions in Healthcare
The collaboration introduces innovative AI technology while ensuring data security and the data you want and actionable insights you need at your fingertips. Tempe, AZ – SDC has partnered with Singular exclusively to integrate secure closed-loop AI capabilities into...
SDC Capture Revolutionizes Clinical Trials with new BYOD ePRO Solution
Phoenix, AZ – Statistics & Data Corporation (SDC), a global leader in life sciencesbiometrics services and technology solutions, announces the launch of itsgroundbreaking Bring Your Own Device (BYOD) electronic Patient Reported Outcomes (ePRO) solution, designed...
